Catalysts are indispensable for many technologies. To further improve heterogeneous catalysts, it is required to analyze the complex processes on their surfaces, where the active sites are located. Scientists of Karlsruhe Institute of Technology (KIT), together with colleagues from Spain and Argentina, have now reached decisive progress: As reported in Physical Review Letters, they use calculation methods with so-called hybrid functionals for the reliable interpretation of experimental data. (DOI: 10.1103/PhysRevLett.125.256101).
Many important technologies, such as processes for energy conversion, emission reduction, or the production of chemicals, work with suitable catalysts only. For this reason, highly efficient materials for heterogeneous catalysis are gaining importance. In heterogeneous catalysis, the material acting as a catalyst and the reacting substances exist in different phases as a solid or gas, for instance. Material compositions can be determined reliably by various methods. Processes taking place on the catalyst surface, however, can be detected by hardly any analysis method. “But it is these highly complex chemical processes on the outermost surface of the catalyst that are of decisive importance,” says Professor Christof Wöll, Head of KIT’s Institute of Functional Interfaces (IFG). “There, the active sites are located, where the catalyzed reaction takes place.”
Precise Examination of the Surface of Powder Catalysts
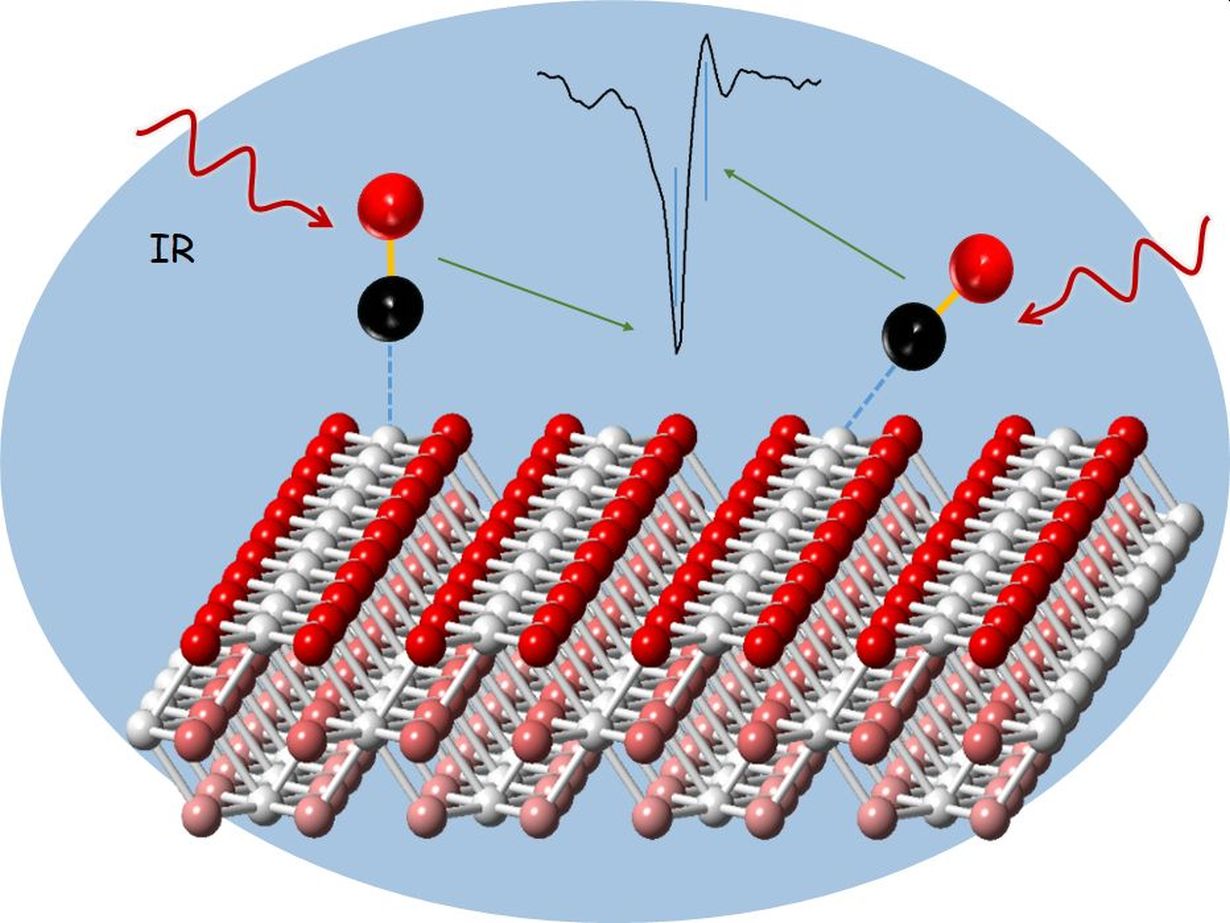
Among the most important heterogeneous catalysts are cerium oxides, i.e. compounds of the rare-earth metal cerium with oxygen. They exist in powder form and consist of nanoparticles of controlled structure. The shape of the nanoparticles considerably influences the reactivity of the catalyst. To study the processes on the surface of such powder catalysts, researchers recently started to use probe molecules, such as carbon monoxide molecules, that bind to the nanoparticles. These probes are then measured by infrared reflection absorption spectroscopy (IRRAS). Infrared radiation causes molecules to vibrate. From the vibration frequencies of the probe molecules, detailed information can be obtained on the type and composition of the catalytic sites. So far, however, interpretation of the experimental IRRAS data has been very difficult, because technologically relevant powder catalysts have many vibration bands, whose exact allocation is challenging. Theoretical calculations were of no help, because the deviation from the experiment, also in the case of model systems, was so large that experimentally observed vibration bands could not be allocated precisely.
Long Calculation Time – High Accuracy
Researchers of KIT’s Institute of Functional Interfaces (IFG) and Institute of Catalysis Research and Technology (IKFT), in cooperation with colleagues from Spain and Argentina coordinated by Dr. M. Verónica Ganduglia-Pirovano from Consejo Superior de Investigaciones Científicas (CSIC) in Madrid, have now identified and solved a major problem of theoretical analysis. As reported in Physical Review Letters, systematic theoretical studies and validation of the results using model systems revealed that theoretical methods used so far have some fundamental weaknesses. In general, such weaknesses can be observed in calculations using the density functional theory (DFT), a method with which the quantum mechanics basic state of a multi-electron system can be determined based on the density of the electrons. The researchers found that the weaknesses can be overcome with so-called hybrid functionals that combine DFT with the Hartree-Fock method, an approximation method in quantum chemistry. This makes the calculations very complex, but also highly precise. “The calculation times required by these new methods are longer by a factor of 100 than for conventional methods,” says Christof Wöll. “But this drawback is more than compensated by the excellent agreement with the experimental systems.” Using nanoscaled cerium oxide catalysts, the researchers demonstrated this progress that may contribute to making heterogeneous catalysts more effective and durable.
The results of the work also represent an important contribution to the new Collaborative Research Center (CRC) “TrackAct – Tracking the Active Site in Heterogeneous Catalysis for Emission Control” at KIT. Professor Christof Wöll and Dr. Yuemin Wang from IFG as well as Professor Felix Studt and Dr. Philipp Pleßow from IKFT are among the principal investigators of this interdisciplinary CRC that is aimed at holistically understanding catalytic processes. For more information on the CRC TrackAct, click https://www.kit.edu/kit/english/pi_2020_106_how-to-make-catalysts-more-efficient.php.
Original Publication:
Pablo G. Lustemberg, Philipp Pleßow, Yuemin Wang, Chengwu Yang, Alexei Nefedov, Felix Studt, Christof Wöll, and M. Verónica Ganduglia-Pirovano: Vibrational Frequencies of Cerium-Oxide-Bound CO: A Challenge for Conventional DFT Methods. Physical Review Letters, 2020. DOI: 10.1103/PhysRevLett.125.256101.
Being “The Research University in the Helmholtz Association”, KIT creates and imparts knowledge for the society and the environment. It is the objective to make significant contributions to the global challenges in the fields of energy, mobility, and information. For this, about 10,000 employees cooperate in a broad range of disciplines in natural sciences, engineering sciences, economics, and the humanities and social sciences. KIT prepares its 22,800 students for responsible tasks in society, industry, and science by offering research-based study programs. Innovation efforts at KIT build a bridge between important scientific findings and their application for the benefit of society, economic prosperity, and the preservation of our natural basis of life. KIT is one of the German universities of excellence.